Why FDA-Cleared IGeneX ImmunoBlots May Finally Solve the Sensitivity Problems in Lyme Disease Diagnosis
For clinicians on the front lines of Lyme disease, a groundbreaking development in Lyme testing has arrived. IGeneX’s IgM and IgG ImmunoBlot test kits have now received FDA clearance – a milestone that promises to transform Lyme disease diagnosis for the better. As a clinician who has treated complex tick-borne illness for over 15 years, I can’t overstate how significant this is. In this article, I’ll break down what the FDA clearance means, how these advanced tests work, and why they address many long-standing frustrations with standard Lyme disease testing. We’ll explore the science in clear terms and discuss how more accurate Lyme testing can lead to earlier, life-changing treatment for patients. Finally, I’ll share how you, as a provider, can deepen your expertise in Lyme diagnostics – because better testing is only part of the solution.
In This Article, You’ll Learn:
-
Why IGeneX’s FDA-cleared ImmunoBlots represent a major advancement in Lyme disease diagnosis
-
How these tests compare to traditional two-tiered testing (ELISA + Western blot)
-
Which antibody bands are highly specific, supportive, or non-specific
-
What clinicians need to know about interpreting results in complex cases
-
How this breakthrough impacts early and chronic Lyme detection
-
Where to get a free Lyme band interpretation cheat sheet for clinical use
FDA Clearance: A Milestone for Advanced Lyme Testing
On July 2, 2025, IGeneX’s new Lyme ImmunoBlot kits for IgM and IgG antibodies gained FDA clearance. The IgG ImmunoBlot kit was cleared in late 2024, and now the IgM kit has been cleared as well. These kits (branded iDart™ Lyme IgM and iDart™ Lyme IgG ImmunoBlots) represent a new generation of serologic testing for Lyme disease. FDA clearance means the tests have passed rigorous review for accuracy and reliability, giving them a stamp of approval that is rare in the Lyme world – a world where many specialized tests have historically been offered as lab-developed tests without FDA review.
FDA-cleared iDart™ Lyme ImmunoBlot test kits for IgM and IgG (developed by ID-FISH/IGeneX) will enable wider availability of advanced Lyme disease testing.
What exactly are these tests? They’re advanced immunoblots that detect whether a patient’s immune system has made antibodies (IgM or IgG) to Borrelia – the bacteria that cause Lyme disease. In essence, they are a modern replacement for the older Western blot step in Lyme testing, but with significant improvements. Each kit contains test strips with a panel of 26 (IgM) or 31 (IgG) distinct Lyme antigen “bands” – far more than any other Lyme blot on the market. These antigens are recombinant proteins representing multiple strains of Borrelia (eight strains, in fact, covering various Lyme-causing species of Borrelia). Notably, the IGeneX ImmunoBlots are the only FDA-cleared Lyme tests that include critical antigens OspA (31 kDa) and OspB (34 kDa). Those particular proteins were omitted from older tests due to past Lyme vaccine concerns, but they are highly specific to Lyme bacteria – and including them helps catch cases that others miss.
From a practical standpoint, the FDA clearance of these IgM and IgG kits means clinicians and labs across the country will soon have access to more sensitive and specific Lyme tests. IGeneX’s lab in California has used the ImmunoBlot as a lab-developed test for years; now, with FDA-cleared kits, other laboratories can adopt this technology and clinicians can have greater confidence in its results. It’s a welcome validation of advanced testing methods that Lyme specialists have been using to get answers for patients. But to appreciate why this is such a big deal, we need to understand how Lyme testing has traditionally worked – and where it has failed us.
The Trouble with Traditional Lyme Disease Testing
If you’ve been diagnosing Lyme disease for a while, you know the standard two-tier testing protocol has serious limitations. For decades, the CDC-recommended approach has been to screen with an ELISA (enzyme immunoassay) or similar antibody screening test and then confirm positives with a Western blot. While this two-tier algorithm is very specific (few false positives), it has poor sensitivity in the early stages of Lyme infection – detecting less than 50% of cases in early localized Lyme.
In fact, one review famously noted that standard Lyme serology was only 46.3% sensitive in early Lyme, meaning that more than half of true cases may be missed (Waddell et al. 2016). In other words, using the old two-tier test for early Lyme is about as good as flipping a coin. As clinicians, many of us have seen this firsthand: patients with clear tick exposure and even classic symptoms sometimes test negative using the insensitive two-tier criteria, especially early on.
Why does this happen? There are several well-documented issues with the traditional Lyme testing model:
- Insensitive screening: The initial antibody screening tests often fail to detect early antibodies. Studies have found that this first test can miss a large fraction of true cases. If that first test is negative, the process stops – and the infection can go undiagnosed. Even newer antibody screening tests (using peptides like C6 or VlsE) improved specificity but still don’t catch everyone.
- Arbitrary Western blot criteria: The confirmatory Western blot requires a certain number of antibody “bands” to be positive (specifically, the CDC criteria call for at least 5 out of 10 IgG bands for a positive result, or 2 out of 3 bands for IgM). These criteria were set in 1994 and were intended for surveillance, not absolute clinical diagnosis. Many true Lyme patients simply don’t produce that many bands – their immune response may be strong to only one or two antigens. If a patient has antibodies to, say, OspA and OspC (two highly specific Lyme proteins) but doesn’t light up 5 different bands, the CDC would call them “negative”. That rigid requirement has led to many missed infections and preventable suffering.
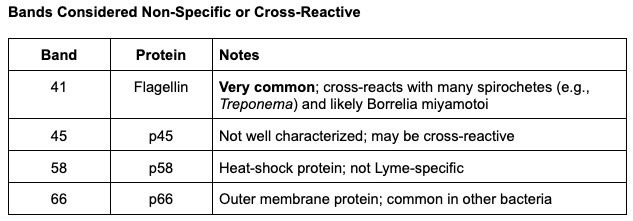
- Non-specific and cross-reactive bands: Some of the bands commonly seen on Western blots (for example, the 41 kDa flagellin band) are not specific to Lyme – they can appear due to other bacteria. Meanwhile, some of the most specific bands (like 31 kDa OspA) were excluded from the CDC criteria entirely. The result is that the standard blot can be both too stringent yet also occasionally misleading (a single nonspecific band is meaningless, but patients and clinicians unfamiliar with the details might misinterpret it).
- Lab-to-lab variability: Reading a Western blot is part science, part art. It involves visualizing bands on a gel or strip – and faint bands might be counted by one lab and not by another. Scoring of blots can be subjective, leading to inter-laboratory variability. Additionally, different labs have used different reference strains or processes, so one lab’s “positive” might be another’s “inconclusive.” All of this adds up to potential confusion and inconsistency.
Let’s dive a bit deeper into the bands
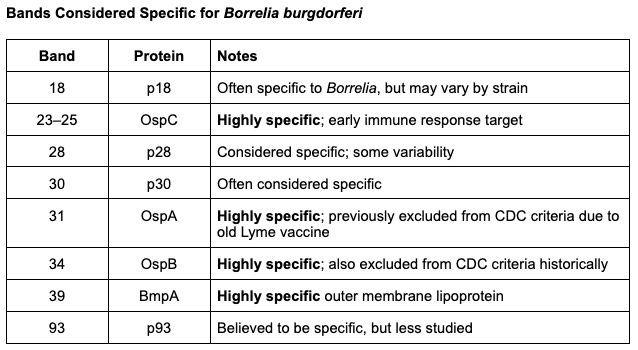
Most specific bands for Lyme IgG: 23 (OspC), 31 (OspA), 34 (OspB), 39
Supportive, but less specific: 18, 28, 30, 93
Nonspecific or cross-reactive: 41, 45, 58, 66
The Bottom Line
The old two-tier system, while specific, has left a huge gap in sensitivity. Experts estimate that tens of thousands of Lyme cases each year have gone undiagnosed or their diagnosis was delayed due to false-negative tests. In fact, Lyme disease has continued to spread – it’s now one of the fastest-growing infectious diseases in the U.S., with an estimated 476,000 people diagnosed annually – yet our conventional testing paradigm was stuck in the 1990s. We desperately needed a better way to identify true cases earlier and more reliably. This is the context in which IGeneX’s ImmunoBlot was developed.
Inside IGeneX’s ImmunoBlot: More Bands, Higher Sensitivity
So, what makes the IGeneX ImmunoBlot different, and why is it considered superior? In a nutshell, it’s a highly multiplexed test that casts a wider net and focuses on truly Lyme-specific targets. Here are the key points:
Comprehensive antigen coverage
The IGeneX IgM and IgG ImmunoBlots each include proteins from 26+ distinct Lyme antigen bands (26 on the IgM blot, 31 on IgG). These antigens come from multiple strains and species of Borrelia, including from Europe and North America. By covering Borrelia burgdorferi plus several related species that cause Lyme disease, the test can detect infections that a single-strain test might miss.
Essentially, it’s one test that can catch many variants of Lyme – important in an era when patients might be bitten by ticks carrying different Borrelia species. Many standard Lyme tests miss true positive patients because they are only looking for Borrelia burgdorferi when we know there are several species of Lyme causing Borrelia in North America alone.
Inclusion of critical antigens (OspA/OspB)
For the first time, an FDA-cleared Lyme antibody test is including OspA (31 kDa) and OspB (34 kDa) on the blot. Why does this matter? OspA and OspB are outer surface proteins of Borrelia that are highly specific to the Lyme bacteria. They were left off standard tests historically (due to an old Lyme vaccine that used OspA, now long off the market), which inadvertently lowered sensitivity. Many Lyme patients have strong antibodies to these proteins. By including OspA/OspB bands and counting them, the IGeneX blot can identify cases that old criteria would have labeled negative.
No other approved Lyme serologic test on the market can detect these OspA/OspB antibodies.
Lyme-specific interpretation criteria
Perhaps the biggest change is how the test is interpreted. The IGeneX ImmunoBlot does not use the outdated CDC band-counting rules. Instead, it uses a new, simplified criterion designed to be both sensitive and specific. According to the FDA-cleared kit instructions, the patient is considered positive for exposure to a spirochete that causes Lyme disease if they have a positive Lyme antibody screening test and a positive confirmatory immunoblot.
The immunoblot is considered positive if antibodies are detected to at least one protein in two or more other antigen groups (ie, 2 Lyme-specific bands are positive). With these advances, now if a patient’s serum shows reactive bands to just a couple of key specific Lyme proteins the test will flag positive.
This is a stark contrast to requiring 5+ bands. In simpler terms: even if a patient’s immune system has produced antibodies to only two or three Lyme-specific antigens, this test can still confirm Lyme disease.
It focuses on quality of bands over sheer quantity. The logic is that if those few bands are known Lyme-specific markers, that is sufficient evidence of infection. This enables more true positives to be identified and treated rather than dismissed.
Improved sensitivity and high specificity
Thanks to the above factors (more antigens, inclusion of specific proteins, and smarter criteria), the IGeneX ImmunoBlot has demonstrated markedly improved sensitivity compared to standard two-tier testing, without sacrificing specificity. Internal studies and independent evaluations showed that the ImmunoBlot could detect a much higher proportion of Lyme cases – “nearly double the sensitivity” of the traditional algorithm, according to IGeneX.
In fact, IGeneX’s immunoblot has been reported to have sensitivity well above 90% in later stages, and significantly higher sensitivity in early infection as well, while maintaining specificity comparable to the standard method. This means it’s catching more of the true cases that were previously missed, yet not falsely labeling people who don’t have Lyme. As the developers put it, “the inclusion of multiple antigens improves the sensitivity of Lyme antibody detection… without sacrificing specificity”.
Objective, reproducible results
The ImmunoBlot uses recombinant proteins printed as lines on a membrane, and results can be read with standardized instrumentation. This reduces the subjectivity of interpretation. The intensity of each line can be measured relative to controls. In short, it’s a more modern, laboratory-friendly assay. It can be run in diagnostic labs with a kit format, improving consistency. The FDA clearance process itself required demonstrating that different labs could get consistent results with the kits – something that will boost confidence among clinicians used to more uniform tests.
What does all this mean for you and your patients? It means that we finally have a Lyme antibody test that is designed to minimize the notorious blind spots of the older tests. Patients who truly have Lyme but only generate a narrow immune response stand a much better chance of testing positive on the IGeneX ImmunoBlot. For example, if a patient’s immune system mostly recognizes OspC (23 kDa) and OspA (31kDa), the ImmunoBlot will catch that and call it positive – whereas the older criteria might have missed it because only one of those (OspC) is in the CDC’s counting scheme.
By requiring as little as two Lyme-specific bands for a positive result, the IGeneX method flags bona fide cases that used to slip through the cracks. This is diagnostically superior for the simple reason that Lyme disease doesn’t always read the textbook – patients don’t always mount five detectable IgG bands. Now, our testing strategy doesn’t insist that they do.
What FDA Clearance Means for Lyme Disease Diagnosis
Beyond the technical improvements, it’s worth reflecting on what this FDA clearance signifies for the field of Lyme disease diagnosis. For years, specialty labs like IGeneX offered more sensitive Lyme tests, but skeptics could dismiss them because they weren’t FDA-cleared. Now, that barrier is coming down. FDA clearance lends credibility and opens the door for broader adoption:
Mainstream acceptance
An FDA-cleared test can be more readily adopted by hospital labs and big reference labs. Clinicians who were hesitant to use out-of-network specialty lab tests may soon have access to similar technology through their usual lab channels. This could increase the overall testing of Lyme disease, especially for patients who might not have been referred to Lyme-literate specialists.
Insurance coverage
Often insurance companies prefer or require FDA-cleared diagnostics. With these immunoblots cleared, it’s possible we’ll see improved insurance coverage for advanced Lyme testing. That can reduce the financial barrier for patients to get accurate tests.
Improved two-tier algorithms
IGeneX’s FDA-cleared ImmunoBlot kits (IgM and IgG) are designed for use in a two-tiered Lyme disease testing framework. But in clinical reality, many patients don’t fit the textbook model.
Providers often encounter individuals who have been symptomatic for months—or even years. In these chronic or late-stage cases, the immune system may no longer produce the robust antibody response seen in acute infection. Yet the traditional two-tier algorithm assumes the opposite: that all patients are producing high enough levels of antibody to trigger a positive result on Step 1 (the initial antibody screening test), which then “reflexes” to Step 2 (Western blot or immunoblot) for confirmation.
But this assumption doesn’t hold up.
Research from Tulane University and others has shown that even patients with acute Lyme infection may not always produce enough antibodies to trigger a positive screening test—let alone those with more prolonged illness (Embers et al. 2017). In other words, the entire testing sequence can fail at the first step, allowing true cases to go undiagnosed.
This is where clinical acumen, combined with more sensitive and highly specific immunoblots, makes all the difference. By bypassing or complementing rigid two-tier protocols, practitioners can use tools like the IGeneX ImmunoBlot to catch true positives that standard algorithms miss—and initiate appropriate treatment sooner.
Confirmation of our approach
For those of us who have been using IGeneX or similar labs, the FDA clearance is a vindication of sorts. It confirms that the science behind these immunoblots is sound. The company had to demonstrate performance to the FDA’s standards – including testing against well-characterized CDC panels, endemic area samples, and cross-reactivity checks. The fact that the FDA reviewers found the test effective and safe speaks volumes. It validates that using a “two Lyme-specific bands is enough” criterion with the right antigens is not only clinically useful, but now officially approved.
Of course, no test – not even this one – is perfect. As clinicians, we must remain vigilant in interpreting results in the context of the whole patient.
The Ongoing Challenge: No Test Catches Everything
Even with the most advanced immunoblots, Lyme disease remains a clinical diagnosis at its core. Indirect tests like antibody assays have inherent limitations that we need to keep in mind:
- Timing and immune response: Early in infection (the first few days to couple of weeks after a tick bite), patients may not have detectable antibodies yet. A very new Lyme infection could still yield a negative result on even the IGeneX ImmunoBlot, simply because the immune system hasn’t responded. Serologic tests work best when some weeks have passed after infection. Thus, a negative test does not fully rule out Lyme – especially if exposure was recent and symptoms are acute or the patient has been sick for many years as they may not be able to mount a robust, ongoing immune response. Clinical follow-up and possibly repeat testing or alternative tests (like PCR) might be warranted in those cases.
- Antibody variability: Some patients, particularly those who are immunocompromised, may not mount strong antibody responses at all. Others might have high IgM but an atypical IgG response, or vice versa. The IGeneX criteria broaden the net, but there will still be occasional true cases that test negative. For instance, a patient might have only one or two strong specific bands with a negative screening test – just shy of the criteria – and still have Lyme. Clinical judgment is needed; if it looks, walks, and talks like Lyme, consider treating or retesting later, even if the test is negative.
- Persistent antibodies: On the flip side, antibodies can linger after infection. IgG can remain positive for years even after successful treatment. The ImmunoBlot can’t distinguish an active infection from antibodies due to past infection – that’s a limitation of any antibody test. We must use history and symptoms to determine if a positive IgG in an asymptomatic patient is indicative of active disease or just past exposure. (Notably, the test’s intended use is in patients with signs/symptoms of Lyme, not as a screening for the asymptomatic.)
- Co-infections and other factors: Lyme often comes with co-infections (Babesia, Bartonella, etc.), which have their own tests and challenges. A patient might feel very ill from a co-infection even if Lyme testing is negative or treated. Advanced Lyme diagnosis, therefore, is not just about one test – it’s about considering the full tick-borne illness spectrum. IGeneX and others offer panels for co-infections, but interpreting those results alongside Lyme serology is an advanced skill in itself.
- False positives: While rare with immunoblots, false positives can occur. Other borrelial infections (like Relapsing Fever Borrelia) might cross-react on a Lyme blot, although IGeneX tries to use species-specific antigens (and they have separate tests for Relapsing Fever). Autoimmune antibodies or other infections might very occasionally cause a band or two to appear. However, the requirement for multiple specific bands makes a false positive highly unlikely. The specificity of the new test was shown to be on par with traditional criteria. Still, as with any test, positive predictive value depends on pre-test probability – in a very low-risk patient, even a positive should be scrutinized carefully.
In summary, the IGeneX ImmunoBlot is a powerful new tool – but it doesn’t replace clinical acumen. It’s indirect evidence of infection. We still have to treat the patient, not just the lab result. The great benefit here is that we now have a test that will support our clinical suspicions far more often than before, giving us actionable information. It’s one more step toward earlier diagnoses and better outcomes.
From Diagnosis to Treatment: Mastering the Next Steps
Having more accurate Lyme tests is a game-changer, but diagnosis is just the beginning. Once you identify that previously elusive Lyme case, the real work begins: effective treatment and management. Lyme disease, especially in disseminated or chronic form, can be complex to treat. Co-infections need to be addressed, Herxheimer reactions managed, and patients often require a blend of antimicrobial therapy (pharmaceutical and botanical) and supportive care. A truly patient-centered, integrative approach is crucial. Many clinicians, understandably, feel uncertainty in navigating advanced Lyme cases – after all, most of us didn’t get comprehensive training in our training on this topic.
That’s why now is the time to level up your Lyme disease expertise. We have better tools like the IGeneX ImmunoBlot; using them to their full potential means knowing how to interpret results in the context of each patient and how to follow through with appropriate treatment plans. If you’re a functional or primary care provider diagnosing Lyme (or suspect you should be diagnosing it more often), ask yourself: Am I fully confident in managing what comes next? If the answer is not a resounding yes, don’t worry – you’re far from alone, and there are resources to help you.
One of the most effective ways to build that confidence and skillset is through mentorship and advanced training. I’ve dedicated my career to not only treating tick-borne illnesses but also mentoring fellow clinicians in this challenging field. That’s why I developed the Lyme Disease Practitioner Certification (LDPC) program – a 12-month training, mentorship and certification course designed specifically to help clinicians master Lyme diagnostics and treatment. In the LDPC program, we dive deep into case-based learning, interpret advanced tests like the ImmunoBlot in real clinical scenarios, and develop personalized treatment strategies for Lyme and co-infections. It’s about turning knowledge into real-world practice with guidance at every step.
If you’re ready to become the Lyme expert your patients desperately need, I invite you to explore the LDPC program. This is a hands-on training where you’ll work directly with me and a community of like-minded practitioners. We tackle the nuances – from choosing the right tests (and labs) for each case, to making sense of results, to initiating treatment protocols that integrate conventional antibiotics with integrative and functional medicine approaches. Most importantly, you’ll gain the confidence to accurately diagnose and treat Lyme disease even in its most complex presentations. The next generation of Lyme diagnostics, like the IGeneX ImmunoBlot, will empower you – and with the right mentorship, you’ll be fully equipped to use these tools to change lives.
In conclusion, the FDA clearance of IGeneX’s IgM and IgG Immunoblots is a victory for the Lyme community and for clinicians committed to better care. It validates what Lyme specialists have long suspected – that we can do better in diagnosing this disease. As we incorporate these advanced tests into practice, let’s continue to stay informed and educated. Our patients are counting on us to be at the top of our game. Lyme disease is often called the “great imitator,” but with cutting-edge diagnostics and proper training, we can unmask it earlier and more often.
Let’s embrace this new era of Lyme disease diagnosis. Equip yourself with the best tools and the knowledge to use them. The suffering caused by missed Lyme cases is preventable – and today, we’ve come one step closer to preventing it. If you’re a clinician passionate about solving complex cases and you refuse to let outdated tests stand between your patients and proper treatment, then join me. Explore the Lyme Disease Practitioner Certification program and become the Lyme-literate provider your patients need. Together, armed with advanced diagnostics and clinical mastery, we can turn the tide on Lyme disease and restore countless lives to health.
References
- Centers for Disease Control and Prevention. Lyme Disease: Clinical testing and diagnosis for Lyme disease.
- Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme Borreliosis. Clin Microbiol Rev. 2005 Jul;18(3):484–509. doi:10.1128/CMR.18.3.484-509.2005
- U.S. Food and Drug Administration. [510(k) Summary] ID-FISH Technology, Inc. – iDart™ Lyme IgG and IgM Immunoblots. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K223898 (accessed July 2025).
- Branda JA, Body BA, Boyle J, et al. Advances in Serodiagnostic Testing for Lyme Disease Are at Hand. Clin Infect Dis. 2018;66(7):1133–1139. doi:10.1093/cid/cix943
- IGeneX, Inc. Understanding the Lyme ImmunoBlot: Advancements in Lyme Testing. IGeneX.com. https://igenex.com/testing/lyme-immunoblot/
- IGeneX, Inc. FDA Clears ID-FISH iDart™ Lyme IgM and IgG ImmunoBlots for Borrelia burgdorferi Diagnosis. IGeneX Press Release. July 2, 2025.
- Branda JA, Linskey K, Kim YA, et al. Two-Tiered Antibody Testing for Lyme Disease with Use of 2 Enzyme Immunoassays, a Whole-Cell Sonicate Enzyme Immunoassay Followed by a VlsE C6 Peptide Enzyme Immunoassay. Clin Infect Dis. 2011;53(6):541–547. doi:10.1093/cid/cir471
- Centers for Disease Control and Prevention. Lyme Disease: Data and Surveillance. https://www.cdc.gov/lyme/datasurveillance/index.html
- Stricker RB, Johnson L. Lyme disease diagnosis and treatment: Lessons from the AIDS epidemic. Minerva Med. 2010;101(6):419–425.
- Johnson L, Wilcox S, Mankoff J, Stricker RB. Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. PeerJ. 2014;2:e322. doi:10.7717/peerj.322
- Waddell LA, Greig J, Mascarenhas M, Harding S, Lindsay R, Ogden N. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of North American research. PLoS One. 2016 Dec 15;11(12):e0168613. doi:10.1371/journal.pone.0168613
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the IDSA, AAN, and ACR. Clin Infect Dis. 2006;43(9):1089–1134. doi:10.1086/508667
- Marques A. Laboratory diagnosis of Lyme disease: advances and challenges. Infect Dis Clin North Am. 2015;29(2):295–307. doi:10.1016/j.idc.2015.02.005
- Fallon BA, Nields JA. Lyme disease: A neuropsychiatric illness. Am J Psychiatry. 1994;151(11):1571–1583. doi:10.1176/ajp.151.11.1571
- Embers ME, Hasenkampf NR, Jacobs MB, et al. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS One. 2017;12(12):e0189071.
- IGeneX, Inc. Lyme ImmunoBlot Kit Package Insert. 2025.


